08 Apr
Learn how Chemius and Allchemist simplify Safety Data Sheets (SDS), chemical compliance, REACH regulations, and PCN/UFI processes. Understand why these tools are essential for regulatory and R&D teams.
When it comes to chemical compliance, there’s little room for error. Whether you’re managing Safety Data Sheets (SDS), navigating REACH regulations, or handling Poison Centre Notifications (PCN) and Unique Formula Identifiers (UFI), the process is complex and highly regulated. At the same time, formulation development and innovation can’t afford to lag behind. That’s where Chemius and Allchemist come in.
At #ECS2025, one question kept coming up:
Why are Chemius and Allchemist separate, even though they use the same product data on the same platform?
The answer is straightforward:
They serve two very different user groups.
Chemius: Built for Chemical Compliance Teams
Chemius is crafted for regulatory and compliance professionals who need to ensure that products meet all legal and documentation requirements. It simplifies demanding processes like:
- Safety Data Sheets (SDS): Automate SDS creation in under 7 minutes, with multilingual support and real-time regulatory updates.
- Labels and Hazard Statements: Generate compliant labels effortlessly, including H and P statements.
- PCN and UFI Management: Submit Poison Centre Notifications and generate UFI codes directly within the platform—minimizing errors and administrative overhead.
- REACH Compliance: Stay updated with the regulatory dashboard and manage changing requirements with ease.
Chemius provides peace of mind by centralizing and automating compliance workflows. You can also create Technical Data Sheets (TDS), perform ADR transport classifications, and connect to various APIs. The Regulatory Dashboard provides early insights into changes in chemical legislation—keeping you one step ahead and helping you reduce the risk of compliance-related fines.

Allchemist: Designed for R&D Teams
On the other side, Allchemist is built with R&D and formulation experts in mind—especially those working in sectors like paints and coatings. It prioritizes technical data critical to product development, including:
- Solids, viscosity, and density
- Raw material descriptions and formulation logic
- Tools to streamline formulation workflows and reduce laboratory testing
Allchemist enables faster innovation without losing sight of compliance.
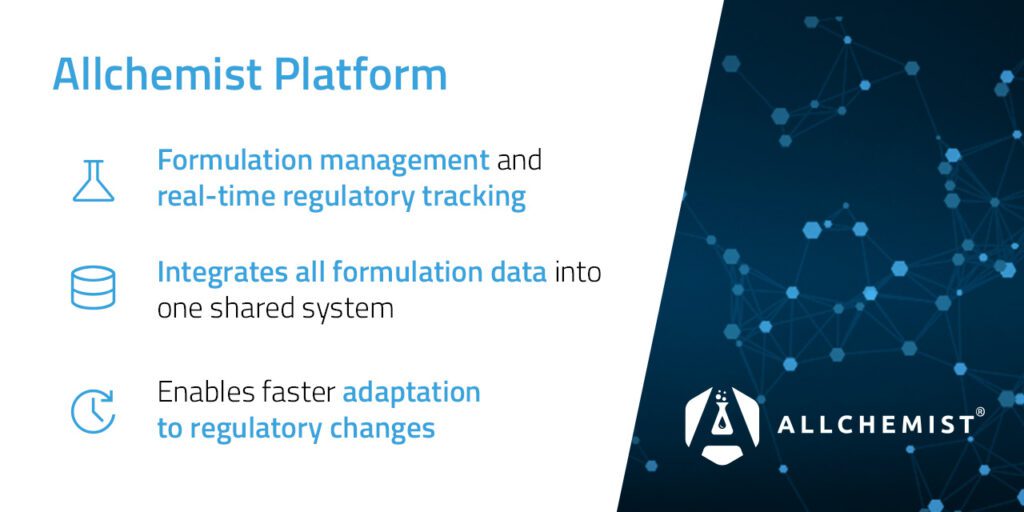


Where They Overlap—and Why Both Matter
Here’s the reality: around 90% of the data used by compliance and R&D teams is the same.
But how that data is used—and presented—makes all the difference.
- An R&D specialist opening Chemius might be overwhelmed by regulatory terms and hazard classifications.
- A compliance manager launching Allchemist could be confused by technical formulation data unrelated to documentation.
That’s exactly why we developed two separate interfaces on a shared platform. Chemius and Allchemist access the same data but present it in a way that aligns with each team’s needs.
Why This Structure Works
Separating Chemius and Allchemist enables better collaboration without overlap or confusion.
And the best part?
👉 You don’t have to choose.
When you sign up for one, you get access to both—at no additional cost.
This way, your R&D team can focus on innovative formulations while your compliance team ensures products are ready for market. Together, they work in sync—bridging the often-siloed gap between innovation and regulation.
How Chemius and Allchemist Help You Stay Compliant


Let’s break down how these tools tackle the most pressing compliance and formulation challenges:
1. Safety Data Sheets (SDS)
Manually creating SDS is time-consuming and error-prone. Chemius automates this process, reducing manual workload by up to 70%. With multilingual support and real-time regulatory updates, compliance becomes efficient and scalable.
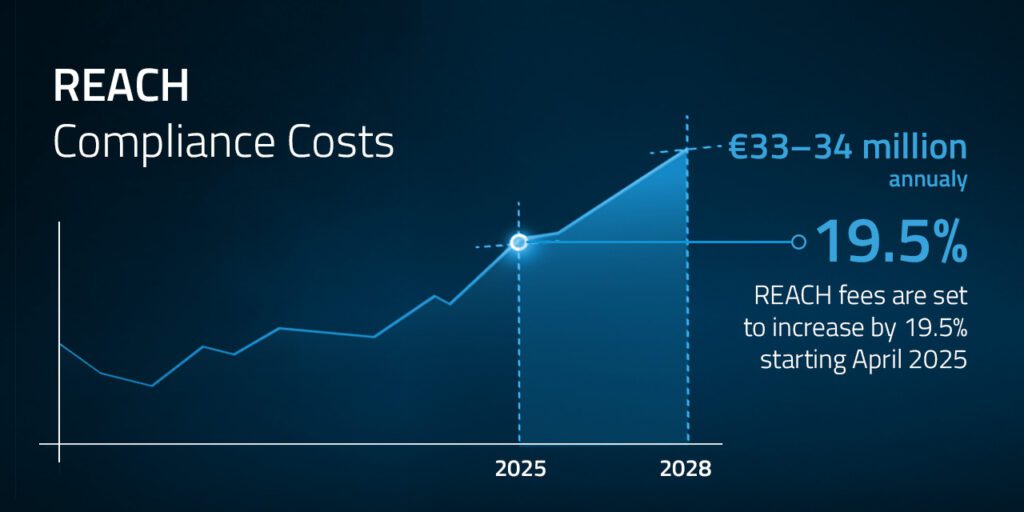
2. REACH Compliance
Keeping up with REACH requirements is demanding. Chemius simplifies this with a regulatory dashboard that flags updates and helps you act proactively.
3. PCN and UFI Management
With Chemius, generating and submitting PCN notifications is simplified into a few clicks. Notifications can be submitted across the entire European Economic Area, saving both time and administrative costs.
4. Formulation Development
Allchemist supports digital raw material management and formulation planning, significantly cutting down on repetitive lab testing. This helps accelerate development and supports environmental goals by reducing material waste.
The Bottom Line
Chemius and Allchemist aren’t competing tools—they’re complementary solutions. Together, they offer a unified approach to chemical compliance and formulation development.
Whether your priority is innovating new products or maintaining airtight compliance, you can work from the same accurate data source with tools made specifically for your workflow.
Ready to simplify your workflow and take control of chemical compliance?
👉 Sign up now and get access to both Chemius and Allchemist—at no extra cost.
Call to Action:
Have questions about how Chemius and Allchemist can support your team? Contact us at connect@chemius.net or call (+386) 41 979 800.


Want learn more about product? Visit www.chemius.net for more information or follow us on LinkedIn. You can also calculate your ROI with Chemius ROI calculator.


