03 Nov

Today I’m going to write about one commonly heard myth that may cause you unnecessary worry. These days you can hear almost on daily basis that every mixture change in composition dautomatically requires a new UFI.
Let me say at the outset that this is not true. Let’s look at the details.
While it’s true that UFI has narrowed some of the composition ranges of substances used in formulation that does not mean every composition change needs new UFI.
There are small changes allowed enabling you to keep the old UFI (avoiding additional unnecessary costs). Small changes refer to:
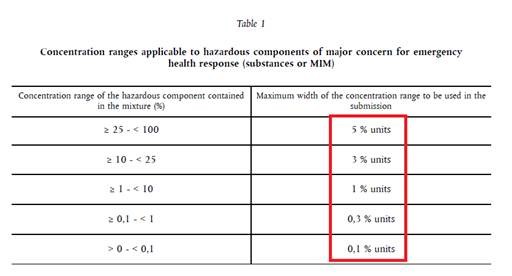
- For substances that are classified with the following H sentences:
H318, H314, H314.1a, H314.1b, H314.1c, H300.1, H300.2 H301, H302, H310.1, H310.2, H311, H312, H330.1, H330.2, H331, H332, H370, H371, H372, H373
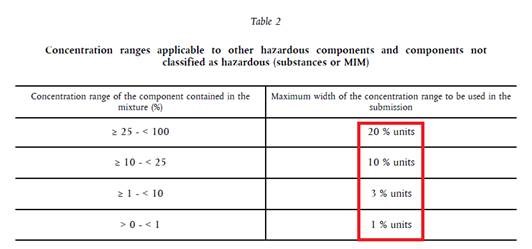
- For other substances (classified with other H sentences or without H sentences):

Now let’s take a look at an example that will illustrate all of this.
Let’s say there’s a 20% substance with H319 in your mixture. When you look in Table 2 above you can see that maximum width range is 10 % units.
What that tells you is that you can use the same UFI if you have 15 % – 25 % of this substance in your mixture. You can play and test formulation within that range and you can still use the same UFI.
However, if the concentration of this H319 substance changes more (if new concentration is less than 15% or more than 25%) than you have to make new UFI/PCN notification.
Now this is just one example showing you how knowing details can save you time and money. UFI brings new opportunities (and threats). Make sure you use them to your advantage. And if you could use some help, we’re here to assist you.



Simona Miklavčič