05 Feb

In 2025, chemical companies face unprecedented challenges in managing product documentation and information. The industry is grappling with a perfect storm of regulatory complexity, data overload, and market pressures that demand both speed and precision.
As our CEO Bojan Buinac stated in his last Linkedin post: “Here’s the brutal reality—especially in Europe. There’s less and less talent in the market. Even fewer people want to work in regulatory compliance. Experts are rare, yet regulations are only getting more complex. It’s not getting better—it’s getting worse.”
Can the industry be saved? Absolutely! How? Automation. Solution? Chemius.
Pain Points Plaguing the Industry with Product information management
Regulatory Compliance and Nightmares with PIM
Chemical manufacturers and distributors are constantly navigating a maze of ever-changing global regulations. Keeping up with requirements across multiple jurisdictions has become a full-time job, and the risks of non-compliance are too costly to ignore.
But every complex problem has a smart solution—you just need to find it.
With Chemius and its regulatory dashboard, staying compliant with EU regulations is no longer a struggle. What once felt overwhelming now feels as effortless as a light summer breeze.
Data Silos and Fragmentation
As businesses expand into new markets and sales channels, they often encounter debilitating data silos. A staggering 80% of organizations struggle with fragmented product information, leading to inconsistencies and errors that impact customer experience and bottom lines.
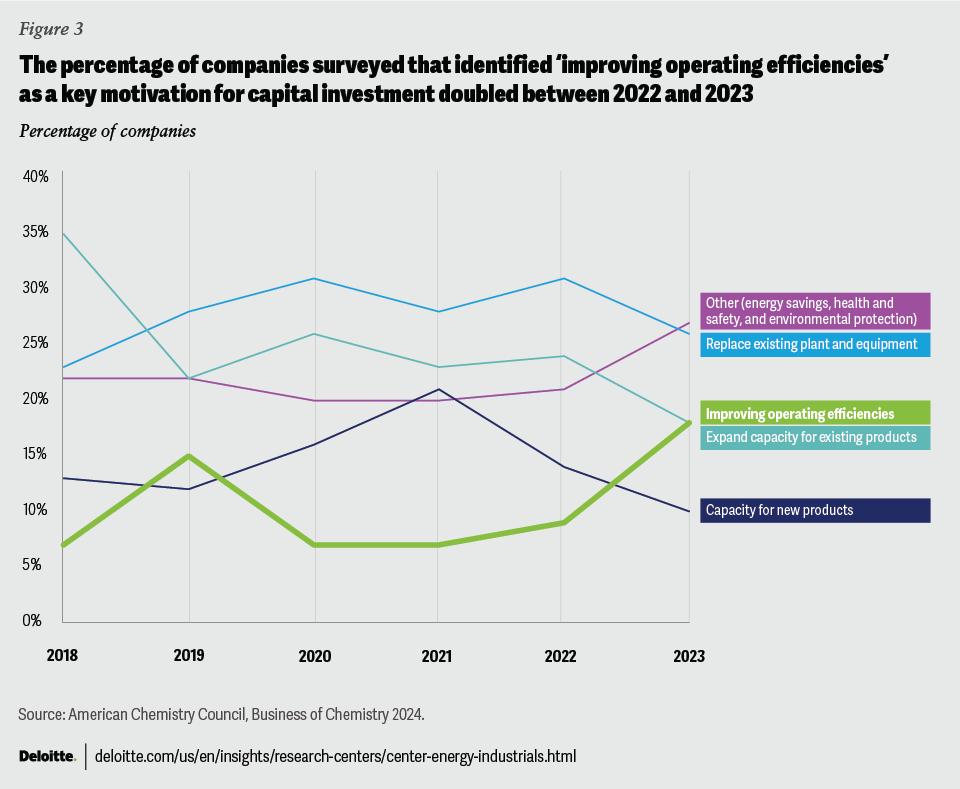
Deloitte wrote an article that stats in 2025, the chemical industry is expected to focus on innovation, sustainability, and resiliency to drive efficiency and growth. In fact, an American Chemistry Council survey of its members, more than 18% of respondents said that the motivation behind their capital investments in 2023 was geared toward operating efficiencies. And yet in 2025 we use PDF for Safety data sheets for data transfering. Not to mention manually researching EU regulations.
Manual Processes Drain Resources
The average office worker spends 10% of their time manually entering data into business applications like ERP systems, PIM and CRM platforms, and even spreadsheets. This isn’t just a minor inconvenience—it’s a hidden drain on productivity and accuracy.
Manual data entry leads to:
- Delays in R&D, slowing down product development
- Late product launches, impacting market entry and competitiveness
- Reduced production output, limiting growth potential
- Customer dissatisfaction, increasing complaints and returns
- Lost sales, affecting revenue and profitability
By automating data processes, businesses can eliminate errors, accelerate workflows, and keep their operations running efficiently—ensuring products reach customers on time and without unnecessary setbacks.
See, in BENS Consulting We’re not here to create every Safety Data Sheet in the world. But we built Chemius, a platform that transforms chemical documentation and formulation management, that helps you create every SDS you need in multiple languages.
With Chemius, companies experience:
- 48% faster formulation development
- 70% fewer manual tasks in SDS and chemical documentation
- Up to 70% cost savings through streamlined change management
And while we don’t aim to handle every SDS out there, Chemius is built to do just that—supporting safety and technical data sheet creation for companies worldwide.
Our CEO Bojan Buinac stated: “If you produce and distribute safety data sheets solely for compliance, then they are definitely a non-value-added cost. You are wasting your time and money on them. However, if you see safety data sheets (combined with Technical data sheets) as a valuable source of data and information for your and your customers’ business processes, in that case, it’s an entirely different story. And because of this story, we developed Chemius.”
Language Barriers Hinder Global Expansion
With the chemical industry becoming increasingly global, the need to manage product information across multiple languages has become critical. Many companies find themselves limited by their ability to efficiently create and maintain documentation in various languages.
We work hand in hand with companies to meet their needs as efficiently and quickly as possible. However, our workforce has its limits, which means some expansions take time.
Currently, Chemius supports 42 languages, and we’re actively working on GHS Revision 7 and US OSHA compliance. Additionally, we’re expanding coverage to more countries based on industry needs.
Cost of Inefficiency
The cost of outdated documentation processes goes beyond lost time. Chemical companies spend hours on manual Safety Data Sheet (SDS) creation—with complex SDSs taking up to 8 hours—while also facing the risk of regulatory fines for non-compliance. The result? Wasted resources, operational delays, and mounting frustration.
In this demanding environment, Chemius provides a smarter way forward. Designed to simplify chemical documentation and Product Information Management (PIM), Chemius helps businesses cut manual work, reduce errors, and stay compliant effortlessly. With powerful automation and flexible pricing, it’s the solution companies need to stay competitive in 2025.
Real Impact on Your Business
1️⃣ Save up to 28 hours per week on compliance documentation (per person)
2️⃣ Ensure 100% regulatory compliance, reducing risks
3️⃣ Integrate seamlessly with existing systems using powerful API connections
Book a demo with us today or reach out at connect@chemius.net, and we’ll send you everything you need—from pricing details to the onboarding process.
Start streamlining your chemical documentation with Chemius subscriptions and experience efficiency like never before.