08 Jul
- Most firms still hunt through spreadsheets to check thousands of substances; a single regulation update can cripple production plans.
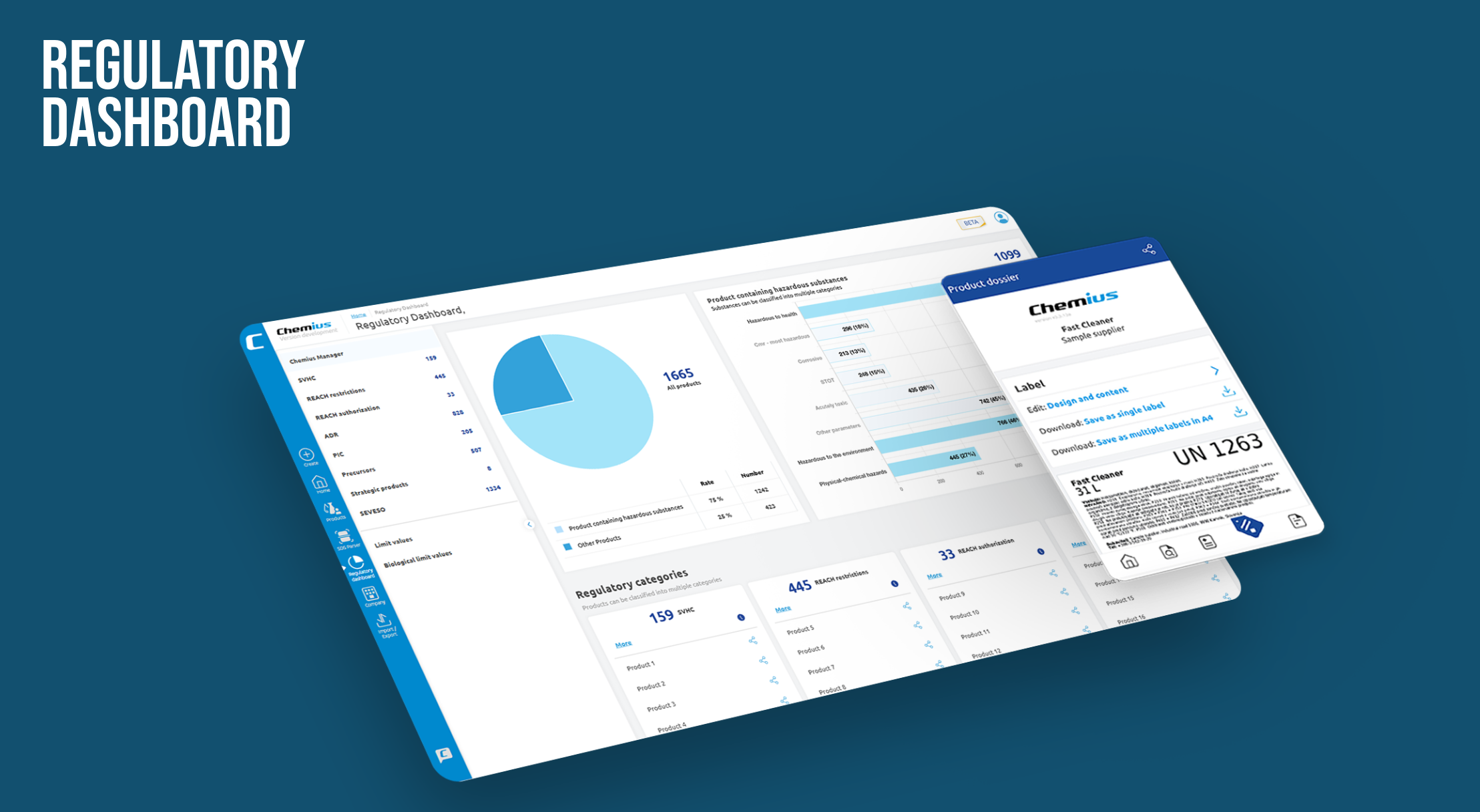
- A Regulatory Dashboard built on structured SDS data flags SVHCs, REACH restrictions, SEVESO chemicals, drug precursors, and more—in two clicks.
- The result is faster decisions, lower risk, and teams free to work on strategy rather than manual checks.
- Case studies show search times falling from days to seconds and audit findings dropping by 90 %.
Introduction
Regulatory lists expand every quarter, yet many businesses still rely on ad-hoc spreadsheets to track compliance. The gap between a published update and your first reaction can expose operations to fines, recalls, and lost market access. This article breaks down why reactive methods fail, how a two-click Regulatory Dashboard changes the chemical compliance game, and what steps you can take today to move from firefighting to confident control.
The Hidden Cost of Reactive Compliance
Why “spreadsheet compliance” breaks down
- Time waste – analysts spend up to 40 % of the week filtering data just to answer “Do we stock this SVHC?”
- Risk blindness – regulations like the Candidate List of SVHCs now contain 247 entries (as of 21 Jan 2025) echa.europa.eu. Manual tools rarely keep pace.
- Decision delays – product launches stall while teams verify whether a new raw material breaches REACH Annex XVII.
- Opportunity loss – competitors that react first secure alternative suppliers and reassure customers early.
Two-Click Compliance: How the Regulatory Dashboard Works
From Safety Data Sheet to actionable map
- Data capture – upload or outsource your SDS library to Chemius. The system parses hazard codes, ingredient CAS numbers, and concentrations.
- Automatic rule-matching – the dashboard checks every substance against live regulatory datasets (SVHC, REACH Annex XVII, SEVESO III thresholds, PIC export rules, ADR transport classes, drug precursor lists eur-lex.europa.eu).
- Visual intelligence – colour-coded pie charts show hazardous vs. non-hazardous volume; bar graphs sort substances by regulatory flag.
- Two-click queries – “Show products containing Candidate List substances” → click; “Filter for SEVESO upper-tier volumes” → click. Answers appear in seconds.
Case study – European automotive supplier
- Inventory: 4 200 SDS files, 18 000 unique ingredients
- Previous method: 3 FTEs, five-day cycle to audit REACH Annex XVII changes
- After dashboard: query time < 30 seconds; compliance reports generated while the team focuses on supplier negotiations
- Result: audit non-conformities cut by 92 % in the first year
What Regulations Must You Track Today?
| Category | Key reference | Why it matters |
|---|---|---|
| Substances of Very High Concern (SVHC) | REACH Candidate List (247 entries, Jan 2025) | Immediate duty to inform customers and, if > 0.1 % w/w, to notify ECHA echa.europa.eu |
| REACH Annex XVII restrictions | EU Regulation 1907/2006 | Legal limits or bans on uses of substances |
| SEVESO III | Directive 2012/18/EU on major-accident hazards eur-lex.europa.eu | Determines tier, reporting, and emergency-planning duties |
| Drug precursors | Regulation (EC) 273/2004 eur-lex.europa.eu | Controls trade of listed chemicals; violations carry criminal penalties |
| ADR transport classes | UN Recommendations, EU ADR agreement | Classifies dangerous goods for road transport |
Best Practices for Proactive Chemical Management
- Centralise SDS data in a single structured repository (Chemius or equivalent).
- Automate cross-checks against live regulatory feeds; avoid static downloads.
- Schedule monthly exception reports so new entries in Candidate Lists trigger alerts before stock arrives.
- Tag substances by production line to see which business units face the biggest exposure.
- Train purchasing teams to run the two-click test during sourcing rather than post-delivery.
- Perform scenario analyses—e.g., “What if Substance X moves from SVHC to Annex XIV authorisation?”—to assess future risk.
Can You Pass the Two-Click Test?
Question: How long would it take your team to list every product containing an SVHC above 0.1 % w/w right now?
If the honest answer is anything over one minute, a dashboard approach will pay for itself quickly.
FAQ
What is the Candidate List and how often does it change?
ECHA updates the Candidate List two or three times per year; it contained 247 substances in January 2025 echa.europa.eu. Companies must track additions or face information-duty breaches.
Does the dashboard work if my SDS files are in different languages?
Yes. Chemius parses GHS hazard phrases and CAS numbers independent of language, then maps them to EU-wide regulations.
How are updates to SEVESO thresholds handled?
Threshold changes are imported nightly from the EU Official Journal. Any product that crosses a tier is flagged automatically eur-lex.europa.eu.
Is drug precursor control relevant outside pharmaceuticals?
Many mainstream solvents and reagents fall under Regulation 273/2004 due to diversion risk. The dashboard highlights both category 1 and category 2 chemicals eur-lex.europa.eu.
What if I only have partial SDS data?
The VIP outsourcing service, Chemius Expert, completes missing sections and imports them so the dashboard can analyse the whole inventory.
Conclusion & Next Step
Reactive compliance drains resources and invites penalties. A two-click Regulatory Dashboard turns laborious checks into an instant, visual scan of every regulatory exposure, freeing your experts to focus on growth instead of paperwork. Ready to see it live? Contact our team for a hands-on walkthrough or start a free ONE MONTH trial. See the pricing list here.


