Safety data sheet
How SDS Authoring Software Transforms Product Information Management (PIM)
- By admin
01 Sep
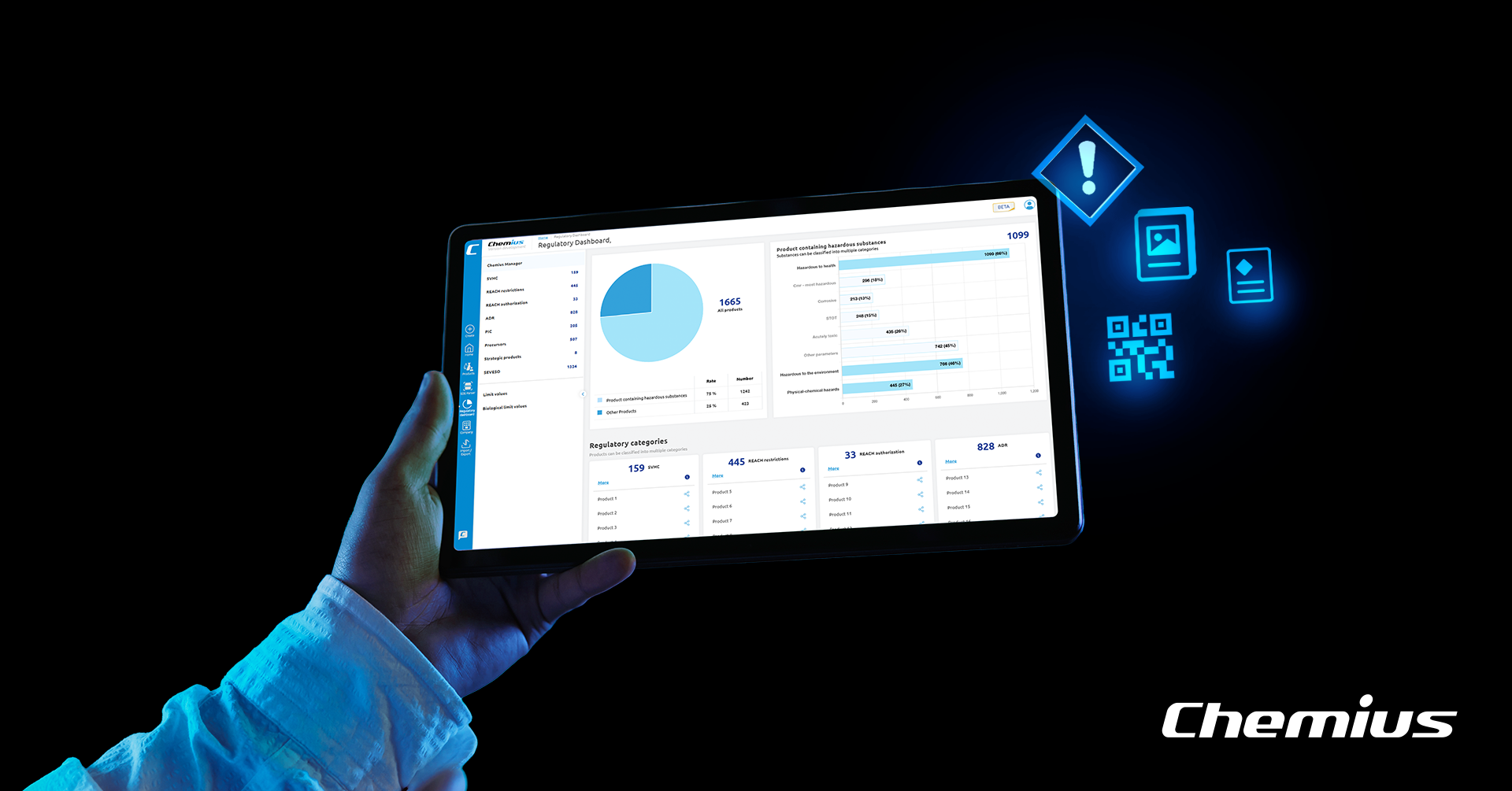
Learn how SDS authoring software integrates seamlessly with product information management, enhancing accuracy, chemical compliance, and operational efficiency.
You could agree that today everyone is facing competitive chemical industry landscape, managing product information is increasingly complex, right? Companies dealing with paints, coatings, aerosols, raw materials, chemical distribution, and automotive products face strict regulatory standards and rising consumer expectations.
SDS authoring software integrated with Product Information Management (PIM) solutions emerges as a pivotal strategy, offering a streamlined approach to managing chemical data while ensuring compliance with global standards such as GHS, OSHA, CLP, ECHA, and REACH.
But what exactly makes the combination of SDS authoring software and product information management indispensable? And why you don’t need them both separetly and spending twice the amount when you can reduce up to 70% compliance costs? Let us break it down for you in this blog down below.
What is SDS Authoring Software and why PIM is essential to be all in one place?
Safety Data Sheet (SDS) authoring software is specialized software designed to simplify creating, managing, and distributing SDSs. Chemius on the other hand offers also other chemical documentation like Technical Data Sheets (TDS), safety instructions, labels, and transport documentations.
By automating compliance processes, your SDS authoring softwares should ensure that chemical product information aligns seamlessly with regulatory demands like Poison Center Notifications (PCN) and Unique Formula Identifier (UFI) codes. Or regulatory areas such as REACH, PFAS, SVHC, SEVESO, precurors, etc, everything what you can find in our Regulatory dashboard.
Integrating separate SDS authoring software into PIM system or vice versa do not fill the gap between regulatory compliance and streamlined data management, and its not enhancing internal collaboration and external communications. Its 21 century and we all should do better in handling chemical complaince.
Why You Don’t Need a Separate PIM with Chemius
Using Chemius eliminates the need for a separate PIM solution because of its built-in capabilities to handle both SDS authoring and comprehensive product information management. With the powerful AI powered SDS assistant, Chemius automatically extracts and populates essential product information directly from your SDS documents, significantly reducing manual data entry.

How Chemius Improves Accuracy and Compliance
Enhanced Data Consistency and SDS authoring Software Accuracy
Having both SDS authoring software and PIM in one platform helps maintain consistency across all product documentation. The seamless synchronisation of data ensures accuracy, eliminating discrepancies that often arise from manual data entry.

No matter what happens, if someone goes to sick leave or the inspector is coming to your wear-house, it’s easy to find all the information about detailed product with a simple QR scan or search inside Chemius.
Streamlined Regulatory Compliance
Chemical compliance is complex, requiring detailed attention to regulations like GHS compliance, OSHA requirements, CLP classification, and ECHA guidelines. SDS authoring software integrated within a PIM structure simplifies compliance by:
- Automating hazard classification and labeling processes
- Efficiently managing UFI codes for PCN compliance
- Ensuring regulatory updates (e.g., REACH, PFAS, SEVESO, or even proposition 65) are applied
Increased Operational Efficiency
Automating processes means significant time savings for regulatory teams and R&D scientists. By leveraging integrated systems, organisations can shift focus from administrative tasks to innovation and product development.
Real-World Application: A Case Study
One of our clients a paint and coatings manufacturer was facing challenges in managing thousands of chemical formulations across multiple regions. They were implementing Chemius as SDS authoring software solution but they wanted also separate PIM that could be combined through APIs, so they can significantly enhanced their ability to comply with international regulations, streamline communication across departments, and drastically reduce the turnaround time for creating and updating documentation. They didn’t need PIM separately, while they could handle all in one platform.
Outcomes:
- 70% reduction in documentation errors and manual tasks
- 48% faster product formulation development time
- Chemius reduces up to 70% of chemical complaint costs
- 40+ languages and TDS PIM communication with their end users
Actionable Tips for Successful Integration



- Evaluate Your Current System: Identify gaps in your existing product information management process, and maybe start using SDS authoring software that also offers TDS modul and other moduls, so you don’t need to have different PIM softwares as well.
- Train Your Team: Equip your regulatory, R&D, compliance, sales, and other teams with comprehensive training.
- Monitor and Optimize: Regularly review system performance to ensure continuous improvement.
Data Comparison: Manual vs. Integrated Systems
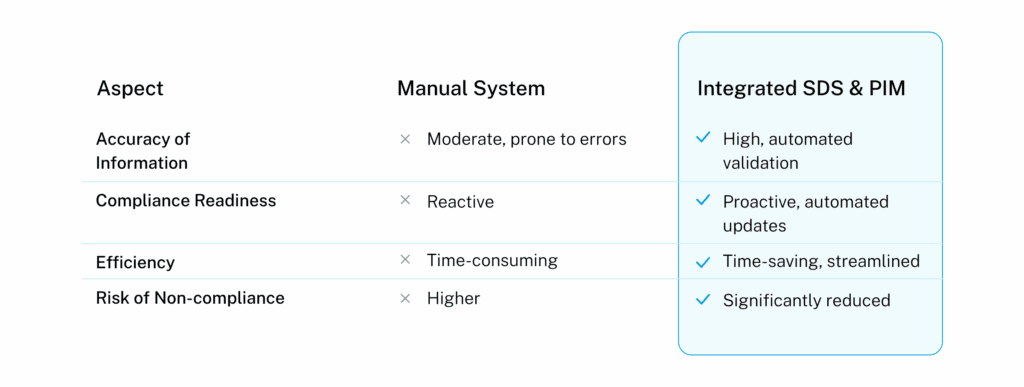


Original Insight
Integrating SDS authoring software not only enhances compliance but strategically positions companies to scale efficiently in competitive markets. Automating data processes allows R&D teams to dedicate more resources toward innovation rather than compliance management.
Summing Up: Why Integration Matters
Integrating SDS authoring software with your Product Information Management system is no longer optional but essential. It streamlines complex regulatory compliance tasks, boosts data accuracy, and significantly enhances efficiency, positioning your organization as a market leader in chemical compliance and product management.
Ready to transform your chemical product information management processes and be chemical compliant?
Contact us today to learn how our solutions can optimize your compliance strategy and operational effectiveness. Book a FREE demo (1,5h) or send us a email to support@chemius.net



Recent Posts
- Top 5 Hazardous Raw Materials in Printing Inks: Navigating REACH SVHC Limits in 2026
- 23rd ATP to CLP Regulation Published – What You Need to Know
- 2026 Chemical Compliance Checklist: Digital Product Dossiers and the Future of Regulatory Readiness – Part 3
- 2026 Chemical Compliance Checklist: PCN, UFI, REACH | Chemius – Part 2
- 2026 Chemical Compliance Checklist: SDS, CLP, GHS, OSHA & LABELS | Chemius – Part 1
Categories
Filter articles by tags
ADR Biocides chemical documentation chemical industry Chemius CLP comliance Digitalization DIgital passport dossier ECHA Endocrine disruptors EU complaint EU Regulation European Chemicals Agency European Green Deal GHS hazards impact innovation Inspectors Label labelling melamine online sales OSHA PCN Poison Centres Notifications Printing Product information management Reach Regulation Safety data sheet Safety data sheet management tool Safety Data Sheets SDS SDS authoring software Software Suppliers SVHC Transport Ufi UK compliance UK compliant UK Regulation UN sustainability development


